Organic chemistry, the chemistry of
carbon-containing compounds plays a huge role in our daily live as well as in
the perfume manufacturing industries.
The most common organic compound for creating
the lovely fragrance in perfumes is ester.
Esters
Esters occur naturally, but can be made in the
laboratory by reacting an alcohol with an organic acid also known as carboxylic
acid. A little sulfuric acid is needed as a catalyst to carry out the reaction.
This is the general word equation for the reaction:
alcohol + organic acid →
ester + water
For example:
methanol + butanoic acid →
methyl butanoate + water
Unsaturated esters have a stronger scent compared to saturated esters
because the higher the molecular weight, the weaker the odor they carry. Thus,
unsaturated ester containing perfume are most highly preferred by manufacturers
when in making of new creation of scents.
What esters smell
like
Different esters have different smells. These are some
examples:
alcohol
|
organic acid
|
ester made
|
smell of ester
|
pentanol
|
ethanoic acid
|
pentyl ethanoate
|
pears
|
octanol
|
ethanoic acid
|
octyl ethanoate
|
bananas
|
pentanol
|
butanoic acid
|
pentyl butanoate
|
strawberries
|
methanol
|
butanoic acid
|
methyl butanoate
|
pineapples
|
Stabilizers and Perfume Bases
All perfumes
are composed of both a base and a fragrance compound. The perfume compound will
account for 20 to 50 percent of the fragrance and is made from essential oils
and synthetic fragrances like from ester. Perfume bases, which account for 50
to 80 percent of a fragrance, are generally made from liquids such as alcohol
and water. They also include a variety of stabilizers, which are used to fix a
perfume's scent and ensure that ingredients do not separate. Common perfume
base is ethyl alcohol and for stabilizers is benzyl benzoate.
Most oil-based perfumes are free of stabilizers. The oils used for these bases are clearly identified and will be familiar to perfume wearers. These fragrances must be applied with the fingertips or with a roller ball and are not available in spray-on form.
Ethyl Alcohol
The majority of perfumes
manufactured from industries contain an ethyl alcohol base which is also known
as ethanol. Also known as ethanol or pure alcohol, ethyl alcohol vaporizes
quickly. It is used in perfume precisely because it acts as a carrier for a
perfume compound but dissolves quickly on the skin. Some organic perfumes use a
denatured alcohol in place of ethyl alcohol due to concerns about the safety of
ethanol-based products.
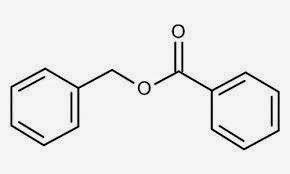
Benzyl Benzoate
Benzyl benzoate is made from the
combination of benzoic acid and benzyl alcohol, is used as a fixative or
stabilizer in many perfumes. This compound is often found in plants and
naturally processed essential oils. When added to perfumes, it allows for the
stability of fragrance ingredients. Benzyl benzoate helps perfumes to have a
consistent scent and can prevent variation in scent profiles for several years.
Benzyl
Benzoate is a natural component of cinnamon essential oil. It composes about 3%
of the oil. Cinnamon essential oil has therapeutic benefit and when used in
small amounts is safe.
Generally the amounts of essential oils used in cosmetics and personal care products is so small that you don't have to be concerned with any precautions associated with the essential oil. The synthetic version, on the other hand is the chemical used to kill head lice and is associated with a variety of adverse effects, including burning and itching of the skin, formation of blisters, reddening, crusting or scaling of the skin, jerking movements, difficulty urinating or sudden loss of consciousness. Thus, try to avoid products with benzyl benzoate listed as an ingredient, if it does not specify that it is from cinnamon essential oil.
Generally the amounts of essential oils used in cosmetics and personal care products is so small that you don't have to be concerned with any precautions associated with the essential oil. The synthetic version, on the other hand is the chemical used to kill head lice and is associated with a variety of adverse effects, including burning and itching of the skin, formation of blisters, reddening, crusting or scaling of the skin, jerking movements, difficulty urinating or sudden loss of consciousness. Thus, try to avoid products with benzyl benzoate listed as an ingredient, if it does not specify that it is from cinnamon essential oil.
Essential Oils
Essential oils are used to add scent to many perfumes. Many of these oils have been sourced from plants, woods, herbs, spices, and other organic materials. However, there are many synthetic essential oils available on the market. Common oils used in the industries are lavender oil, jasmine oil, opium oil and more.
Essential oils are used to add scent to many perfumes. Many of these oils have been sourced from plants, woods, herbs, spices, and other organic materials. However, there are many synthetic essential oils available on the market. Common oils used in the industries are lavender oil, jasmine oil, opium oil and more.
Ethers are much stronger
than esters but both have similar properties. They are a powerful
antispasmodic, antibacterial, and anti-inflammatory. They are very gentle on
the skin and particularly efficient in relaxing and rebalancing the nervous
system. Examples of ethers and esters include cinnamyl acetate which are found
in cinnamon, and myrtinyl acetate found in myrtle.
Conclusion
There are many esters or
chemical elements used in making a bottle of perfume such as benzyl acetate,
diethyl phthalate and so on. Some but not all synthetic perfumes are safe for
use as some chemical containing perfumes such as the benzyl benzoate have some
negative effects on health. So when choosing for a good and safe bottle of
perfume, always check the ingredients listed behind the bottle to avoid the
danger. Like how many always say, “Better safe than sorry.”

No comments:
Post a Comment